
Messege From
the President
We are delighted that you are here to learn more about our company! Tasnim Pharma is nicely positioned to take full advantage of the opportunities that the Iranian healthcare industry offers!
Since our inception in 2010 we have been constantly investing in further improving our organizational capabilities and in creating Tasnim Pharma as a leading manufacturer of quality medicines in Iran.
I invite you to join us in our exciting journey as Tasnim Pharma brings new products and new therapeutic approaches to the Iranian patients.
Thank you for your continuing support.
By Abbas Movahednia



In our state-of-the-art facility we manufacture a broad range of prescription and OTC products as well as vitamins and supplements. We are actively expanding our product portfolio, complementing our in-house development with a targeted licensing-in strategy which will also take Tasnim Pharma into new therapeutic areas. We currently market our products in over 8,000 retail pharmacies and in 500 hospitals. Given the quality of our products we are now also selling Tasnim Pharma brands in three surrounding regional markets.
Our mid-term strategy aspires to place us among the leading Iranian pharmaceutical manufacturers as we strive towards our goal of reaching the first 100 million dollars in sales. Significant investments continue to be made as we strategically reposition ourselves to meet the challenges of the future.

of Success The Story
Construction
In 2008, the analytical reagent facility was acquired and Dr. Abbas established Tasnim Pharmaceutical Co. During that time, Tasnim Pharma was considered as one of few pharma company from private sector in Iran. From mid of 2008, the construction of the first GMP-certified production line for solid oral dosage forms was started.

First sales
By successful completion of manufacturing facility the first batch of prescription products targeted for the cardiovascular diseases was launched. Despite the tough competition in this therapeutic segment, the quality and novel packaging of the launched products well-positioned Tasnim Pharma among cardiologists, and this contributed significantly in increase of company revenue within a short period of time.

Launch of supplements
In 2012, Tasnim Pharma started to manufacture niche supplement products targeted for elderlies and aged populations and the company officially expand the product portfolio to supplement products.

Second GMP Facility
Market leader in two therapeutic area
By increasing the sales of company, Tasnim board members decided to establish the second GMP facility in 2013. The new facility which was equipped with 5 blistering and capsule filling was commissioned for hard-gelatin capsules added as extension to previous facility. In 2013, by reaching the number of branded products to more than 26, company decided to re-structure the sales and marketing team and established 5 dedicated business units in selected therapeutic areas. This unique marketing and sales approach positioned Tasnim Pharma as market leader in urology and gastroenterology segment.

Commenced Soft-gelatin facility
Closing top-notch international in-licensing deals
The company put together a growth strategy for the next 5 years and decided to be the first Iranian company with International standards. Tasnim has decided to team up with leading international players and the company could conclude 4 international in-licensing deals with well-known EU and Australian based companies. At the same year, Tasnim has opened two regional sales offices in neighborhood countries, and started to market more than 35 branded generic in these markets. In 2016, the company identified soft-gel products as an area for company expansion and commissioned it’s first soft-gel facility in August 2016. The dedicated soft-gel facility is manufacturing niche prescription products.

Contract manufacturing launch of soft-gel
From 2015, Tasnim Pharma was entered into a CMO agreement with two UK-based company and commenced contract manufacturing of supplement and prescription products under the respective partners. In the same year, Tasnim has announced annual nation-wide award to top university scholars and research groups in Iran. This award was aimed to help the pharmaceutical research societies and motivate the university scholars for innovation in pharma industry.

Unique production facility
On 2020, the company started high potent products in form of soft-gelatin in the market. A dedicated unique facility in its-own type in Middle East. The products from Tasnim is registered in surrounding countries and Russia.



Our Vision
A large and a growing population of over 80 million people and a relatively widespread acctess to healthcare services make Iran an attractive pharmaceutical market in the MENA Region. Even though the pharmaceutical and healthcare sectors are not affected by sanctions, the Iranian economy is expected to return to positive growth in 2016. With sanctions relief on the way, the Iranian economy is forecasted to show real GDP growth over the next 5-7 years estimated to be annually 6-8%.
Today, the local industry covers 90% of the needs of local market requirements; however, Iran is still dependent on the importation of specialized medicines. State owned companies control an estimated 80% of the pharmaceutical market; however, the private companies are gradually starting to get market share as a result of the Ministry of Health incentives and encouragement for the local production of quality pharmaceuticals. Today, the pharma industry is also beginning to export its products to the surrounding regional markets.
Given the market developments, Tasnim Pharma’s vision is to become a world-class pharma company with its roots in Iran and a key player in designated therapeutic areas.
Our Mission
We aspire to be a world-class pharmaceutical company. Our corporate vision is driven by innovation, integrity and social responsibility. We aim to improve the lives of patients who take Tasnim Pharma’s medicines

INNOVATION
We develop best-in-class prescription medicines, OTC brands and supplements Using our in-house intellectual skills and strategic insights, we adapt global know-how to local solutions

SOCIAL RESPONSIBILITY
We are responsible and accountable for our actions. We create an internal environment which fosters openness and respect for the needs of our stakeholders and partners. We respect the physical environment that we operate in and strive to reduce waste in our operations

INTEGRITY
We strive to provide the latest scientific information to the medical and healthcare professions in a timely manner. Risks and benefits are presented in a balanced manner. We build trust among our stakeholders
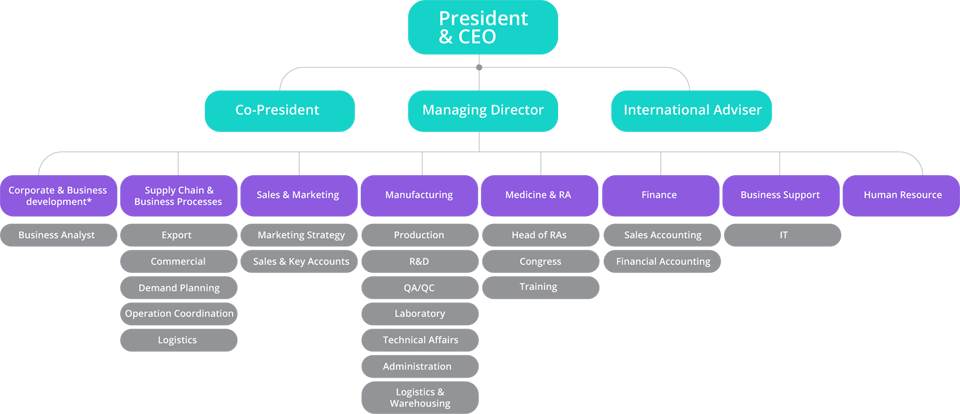
Meet Our Professions
“Build Mutually Rewarding Partnerships”
We establish long-term partnerships with win-win outcomes.
Win-Win Strategy
We work closely with our partners to bring our products to market as quickly as possible. We have established a regulatory pathway which aims to reduce the approval lead-times for our products.
We have an experienced team of well-trained key account executives and medical representatives. Using our established network of distributors, our unique market access model ensures that our approved products get to the right customers at the right time.
We are constantly looking to strengthen our product portfolio through carefully selected partners who share our vision and help further improve our innovation capabilities
Tasnim Pharma is developing mutually rewarding and long-term relationships with our partners. Our partnerships are focused on integrity, mutual respect, and transparency and with the objective of developing innovative, effective and quality treatments for our patients.
We have an experienced business development team which is constantly on the lookout for new leads, collaboration options, and mutually rewarding partnerships.
Our wealth of international experience, our ability to work closely with partners and our flexibility in structuring win-win deals has enabled us to establish multiple and sustainable partnerships. We successfully market both proprietary and in–licensed products from our multiple European partners.







