The role of intestinal microbiota in the treatment of various diseases
During the past decade, research into the human gut microbiome has accelerated rapidly, revealing that the microbiota that inhabits multiple body niches — particularly the gut — have an intricate relationship with human health. The excitement around this microbiota and its genomic composition (known as the microbiome) and its influence on human health and disease has extended to every area of medicine, including neurology. Autoimmune and inflammatory conditions and their animal models — in particular, multiple sclerosis (MS) and its animal model experimental autoimmune encephalomyelitides (EAE) — were among the first diseases in which microbiome research demonstrated important basic concepts and began to suggest novel avenues for treatment.
In this Review, we summarize current knowledge of how the gut microbiome is involved in neuroimmunological disease, focusing on autoimmune demyelination and MS. We first outline how the microbiota influences immune function through complex interactions in the gut. We then discuss the evidence that dysbiosis is associated with MS and consider how the microbiota could be targeted therapeutically. Microbiota and the immune system, Neuroinflammatory diseases such as MS involve immune dysfunction, and alterations in the microbiota can influence immune function in multiple ways. First, Mbacterial metabolites influence the intestinal production of serotonin, which itself regulates the function of many immune cell types.
In addition, through complex interactions with several components of the immune system, the gut microbiota make a major contribution to the regulation of host T cell and B cell maturation and activity5. In turn, these lymphocytes regulate the microbiota through the maintenance of the intestinal barrier and low-grade microbial translocation to other body sites. These interactions begin at the intestinal epithelium, which commensal (and pathogenic) bacteria access by breaking through the mucus layer. This mucus layer is different in the small intestine (which has a single, tightly attached layer of mucus) and in the colon (where the mucus is organized into a loose outer layer and a denser, firmly attached inner layer)6, with implications for the composition and function of immune cells that are resident in the gut-associated lymphoid tissue in these two regions — pro-inflammatory lymphocytes.
whereas anti-inflammatory lymphocytes predominate in the colon. Signals from the microbiota create complex interactions between epithelial cells, dendritic cells, macrophages, and innate lymphoid cells. Normally, these interactions are tightly controlled by innate and adaptive immune responses. However, a breakdown of intestinal homeostasis owing to dysbiosis can result in dysregulated systemic immune responses. Given that systemic immune processes can contribute to neuroinflammation in MS and other neuroinflammatory disorders, this dysregulation can contribute to and/or exacerbate neuroinflammation. In the following sections, we discuss in detail how the microbiome influences immune function and how studies in mice and humans have demonstrated that these effects are important in neuroimmunological disease.
Serotonin
Serotonin — also known as 5-hydroxytryptamine (5-HT) — has well-known, critical roles in the brain andgut. In the brain, it acts as a neurotransmitter involved in behaviour, cognition and locomotor activation, and in the gut, it influences intestinal peristalsis, motility, secretion of mucus, vasodilatation and absorption of nutrients. However, serotonin and its metabolites (for example, N-acetylserotonin and melatonin) also affect immune regulation via receptors expressed on innate and adaptive immune cells.
Approximately 90% of all serotonin production occurs in the gut, and the microbiotastrongly influence this production. Consequently, the microbiota can influence immune function indirectlyvia their effects on serotonin production Microbiota and serotonin production in the gut. Serotonin is produced by the metabolism of dietary tryptophan. This metabolism is mediated by the enzymes 5-hydroxytryptophan decarboxylase, tryptophan hydroxylase 1 (in the enterochromaffin cells of the gut) and tryptophan hydroxylase 2 (in the brain19). Animal studies have demonstrated that the gut microbiota can influence the amount of serotonin synthesized by enterochromaffin cells by modulating expression of tryptophan hydroxylase 1.
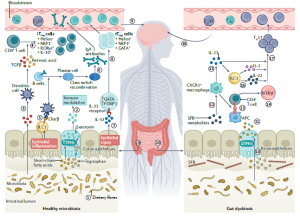
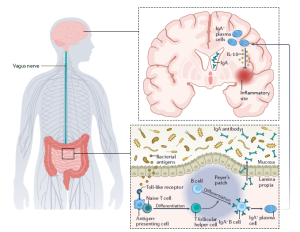
The gut microbiota contributes to the modulation of neuroinflammation. In healthy individuals (left), commensal and anti-inflammatory bacteria interact with the colonic mucosa to maintain homeostasis. Bacteria that are part of the commensal gut microbiota produce short-chain fatty acids through the fermentation of dietary fiber. These short-chain fatty acids increase the activity of tryptophan hydroxylase 1 (TPH1) in enterochromaffin cells of the gastrointestinal epithelium, which increases the production of serotonin from dietary tryptophan. Serotonin regulates the secretion of cytokines (such as tumor necrosis factor (TNF), the interferon IFNγ and the interleukins IL-1β and IL-17) by immune cells, mediates recruitment of neutrophils and activation of T cells, and can activate autoreactive T cells to produce IL-6 and IL-17. Signals from the microbiota also have direct effects on immune processes. Short-chain fatty acids can activate dendritic cells that release TGFβ and retinoic acid, which promote differentiation of CD4+ T cells into intestinal-inducedT regulatory (iTreg) cells). Inflammation of the epithelium induces the production of lymphotoxin α and β (LTα/β) by type 3 innate lymphoid cells (ILC3s), which leads to the activation of dendritic cells. thereby contributing to the development of plasma cells, which undergo class switch recombination to produce immunoglobulin A (IgA) antibodies. In addition, epithelial cell injury causes the release of IL-33.
recirculation of IgA-producing plasma cells in MS. Bacterial antigens activate antigen-presenting cells, which lead to the differentiation of naive T cells into T follicular helper cells. These cells in turn promote the differentiation of B cells into IgA+ B cells and plasma cells that produce IgA antibodies. The plasma cells and antibodies enter the circulation and reduce inflammation in the brain owing to IL10 release by the plasma cells.
Microbiota and Obesity
Gut microbiota is an effective factor in obesity control by the nervous system. A study on mice with a special diet to gain weight shows that the number of Lactobacillus species decreases as the mice become obese. Gut microbiota is directly related to the feeling of satiety. Intestinal microbiota by fermenting complex carbohydrates into short-chain fatty acids causes a feeling of satiety. Short-chain fatty acids reduce appetite by changing the levels of glutamate, glutamine, gamma-aminobutyric acid (GABA), and neuropeptides. Acetate produced by intestinal microbiota causes physiological and regulatory changes in the hypothalamus and appetite suppression. By fermenting food into acetate, propionate, and butyrate, intestinal microbiota produces intestinal hormones such as tyrosine peptide, tyrosine peptide, and glucagon-like peptide. Peptide YY and glucagon-like peptide affect the hypothalamus and cause a feeling of satiety.
Microbiota and learning and memory
Gut microbiota can even be an effective factor in learning and memory. Studies show that the intestinal microbiota also affects the spatial memory of mice. Rats treated with ampicillin are impaired in their spatial memory. Rats treated with ampicillin show poor performance in object recognition memory tests. It is expected that the intestinal microbiota in humans is also effective in memory and learning. Bifidobacterium breve, Lactobacillus fermentum, and Bifidobacterium longum strengthen memory as probiotic treatment. Intestinal microbiota by affecting the amygdala of the brain causes changes in emotions, and increases the power of memory and learning along with emotion, social behaviors, and anxiety. The amygdala is a part of the limbic system of the brain that plays an important role in learning (especially learning with emotion), and understanding emotions. , responses to pain, happiness, and fear. Gut microbiota can change learning and memory by affecting the hippocampus of the brain. Recently, it is said that gut microbiota regulates hippocampal neurogenesis. A study on microorganism-free mice shows that the gut microbiota can induce the expression of many miRNAs and mRNAs in the hippocampus.
The effect of antibiotics on microbiota and behavior
One of the ways to regulate gut microbiota and brain function is to use antibiotics. Antibiotics cause many changes in the intestinal microbiota, but the regulation of the microbiota must be precise and controlled. Using antibiotics to regulate intestinal microbiota increases Actinobacteria, and Firmicutes and decreases Bacteroidetes. Rifaximin increases Lactobacillus species such as Lactobacillus casei. On the other hand, antibiotics and antimicrobial substances cause dysbiosis of intestinal microbiota and change behavior, and increase anxiety and stress. . In sum, all the evidence shows that a disturbance in the balance of gut microbiota causes brain dysfunction and behavior. Although antibiotics can be used to regulate intestinal microbiota, antibiotics are an important factor in disrupting the balance of intestinal microbiota. If the change in brain function and behavior is attributed to the penetration of antibiotics into the brain, studies show that antibiotics that do not enter the brain also cause changes in brain function and behavior. Antibiotics ampicillin and vancomycin disrupt the balance of intestinal microbiota and then cause memory disorders, cognitive disorders, and memory in mice. Antibiotics can change about 30% of the gut microbiota. The microbiota may return to its original state over time, but it usually does not recover completely. Antibiotics have a negative effect on gene expression, protein activity, and intestinal microbiota metabolism. Today, excessive use of antibiotics is a global challenge and not only causes bacterial antibiotic resistance but may also affect brain function and human behavior and mood.