Timeline showing the development of drug products for the treatment of inflammatory bowel diseases. The name of the drug are indicated in bold and brand name(s) of the product are listed below

Timeline of the evolution in the design of colonic drug delivery systems with key technological examples
1-pH-dependent delivery systems
The pH-dependent approach to drug delivery utilises the innate pH changes along the gut. The pH of the GI tract shows variations between and within individuals and is highly affected by food intake. Whilst the lowest pH range lies in the stomach region (pH 0.4 – 4.0 in the fasted state and pH 2.0 – 4.5 in the fed state), pH begins to rise in the proximal sections of the duodenum (pH 5.0 – 7.0). Moving from the jejunum (pH 6.6 ± 0.5) down through to the ileum, GI pH peaks at 7.5 ± 0.5 . In the colon, pH drops to 6.4 ± 0.6 in the cecum and begins to gradually increase down the GI tract, reaching 7.0 ± 0.7 in the rectum. Dietary carbohydrate substrates that are indigestible to human enzymes are converted by colonic bacteria into short chain fatty acids (SCFAs) which lower pH in the proximal colon . The pH increase from the proximal to the distal colon is most likely due to reduced production of bacterial SCFAs. Variations in GI pH between and within subjects do not just affect humans, but are also apparent across other species. As such, variability does not only affect the way a formulation behaves when taken by a patient, but also poses limitations when assessing efficacy in animal models. Thus, not all animal species are representative of human physiology, making some unsuited for simulating human in vivo conditions in preclinical studies.
In particular, pre-clinical rat models may not be suitable to simulate the human colonic lumen for bacterial metabolism and drug stability measurements . Taking this into account, it is possible to exploit the pH variations along the GI tract for targeted drug delivery. By coating formulations with an enteric polymer that disintegrates or dissolves in response to changes in the GI pH, drug release can be targeted to specific regions of the gut. Film coatings used for this type of targeting should start dissolving at a pH of around 6 – 7, and be sufficiently thick to ensure that drug release is delayed until the colon is reached (an enteric film coating needs time to dissolve after its threshold pH value is only slightly exceeded).
The polymers used should be insoluble under the acidic conditions of the stomach and the proximal regions of the small intestine. In reality, most pH-mediated mechanisms release their drug cargo from the terminal ileum to the colon; as such, their delivery can be defined as ileo-colonic [59]. Examples of such pH-sensitive polymers include anionic co-polymers of methacrylic acid and methyl methacrylate . (e.g., Eudragit_ L, pH threshold of 6; and Eudragit_ S, pH threshold of 7 from Evonik, Germany). In general, these polymers have free carboxylic acid groups which remain unionised in acidic conditions, but become deprotonated once exposed to a neutral environment, rendering the macromolecules more hydrophilic and triggering their dissolution.
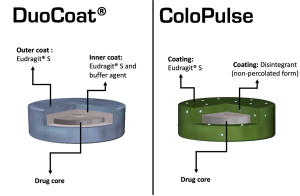
Graphical illustrations showing the colon targeting strategies of the DuoCoat_ and ColoPulse systems.
DuoCoat_ incorporates two pH-sensitive coating layers; the inner is composed of partially neutralized Eudragit_ S combined with a buffer agent [e.g., citric acid/KH2PO4/(NH4)2CO3], and the outer of solely Eudragit_ S . Compared with single-layer pH-dependent coatings, double-layer systems often achieve a shorter dissolution lag time and higher drug release rates once the solubility pH threshold is reached . In the case of DuoCoat_, the polymer coating can dissolve from both its inner and outer surfaces. As intestinal fluid gradually penetrates through the outer coating, the inner coating rapidly dissolves and produces a dynamic internal environment of high buffer capacity, expediting the dissolution of the outer polymer .Another attempt to improve upon pH-mediated delivery includes the ColoPulse system, which as the name suggests, aims to achieve ileo-colonic delivery via pulsatile drug release .
The system comprises a disintegrant (e.g. sodium starch glycolate, croscarmellose sodium, microcrystalline cellulose or alginic acid) incorporated in a pH-sensitive polymer (e.g. Eudragit S) in a non-percolating form (where no continuous network of disintegrant particles is created). Once the pH threshold of the polymer is reached, considerable amounts of fluids penetrate the coating. The presence of the disintegrants accelerates the disruption of the film coating, and a more pulsatile drug release profile is achieved. A potential limitation of the ColoPulse system, however, is that an organic-based coating is required to avoid premature swelling of the superdisintegrant.
2-Time-dependent delivery systems
Time-dependent systems attempt to utilize the time delay between dosage form ingestion and colonic arrival to achieve colon-specific targeting. However, whole gut transit is highly variable, whereby movement through regions occurs at intervals . In the colon, rhythmic contractions mix intraluminal contents and can propagate into high-amplitude propulsions that move contents in defined bursts, enabling defecation . However, several factors play a role in gut motility and transit. An example of such is biological sex. Females are reported to have significantly delayed gastric emptying, and longer whole gut and colonic transit times compared to males . In terms of differences in the residence times within regional colon sections, females may have longer transits through the transverse colon and descending colon, but shorter transit in the rectosigmoid part . These sex differences have largely been attributed to the interplay and action of endogenous hormones, though further research is needed to validate and quantify these effects . Age and body mass index (BMI) also have significant effects on GI motility and transit. Increasing age is associated with longer whole gut, colonic, ascending colon, transverse colon and total right colon transit times, but shorter rectosigmoid times . Age-associated risk factors for slow colonic transit include polypharmacy (i.e., the concomitant use of five or more drug products), decreased fibre intake, and lower levels of physical activity . Increasing BMI has been shown to decrease whole gut transit and gastric emptying time, and potentially increase colonic transit time . Rapid gastric emptying has the propensity to reduce negative feedback satiety signals, thus increasing the risk of overeating . Less is known about the relationship between colonic motility and obesity.
The time-based formulation approach for targeted drug deliveryto the colon relies on average GI transit times. Despite the high fluctuation and inconsistency in the gastric residence time, time-dependent systems should remain completely intact when in the stomach region. Once in the duodenum, the rise in pH may initiate a lag phase but no major drug release should occur. This triggering signal can be achieved through the application of an outer enteric coating, whose dissolution should be equivalent to or surpass that of the average small intestinal transit time . Theoretically, such an effect can be achieved using any approach that results in a predefined delay interval. Regardless of the strategy used to delay the drug release, these systems should have a consistent lag phase and reproducible effect. To ensure that, sealing plugs or layers composed of swellable and erodible polymers are often used to isolate the drug-laden regions or to cover the interior of the drug reservoirs . In fact, the majority of time-based dosage forms intended for colonic drug delivery were initially developed for chronotherapy in the form of pulsatile drug release .Systems for time-controlled colonic targeting can conveniently be grouped as reservoir, capsular and osmotic devices. Reservoir systems can in turn be differentiated based on the functional characteristics of their coating layer, which may function as a rupturable, erodible or diffusive barrier. Reservoir systems with a diffusive membrane represent one of the first attempts to design a time-dependent drug delivery system. Several examples are reported in the literature, mainly dating back to the late 1990s. In these systems, the formulation trigger phase coincides with full water penetration through the diffusive external layer, occurring after upper GI transit. For instance, one study proposed a system in which the drug was incorporated into a core formulation and subsequently coated with a mixture of Eudragit_RS and channelling agents (e.g. NaCl and Emdex_ binders. Drug release from these dosage forms should be delayed until the channelling agents are dissolved, creating diffusion pathways for the drug. The type and particle size of the investigated channelling agents as well as the composition of the core are reported to influence the lag time and release rate of the drug. Gazzaniga and colleagues suggested a coating of immediaterelease 5-ASA tablets with two layers: An inner coating based on a low-viscosity hydroxypropyl methyl cellulose (HPMC), and an outer coating based on Eudragit_ L30D (Fig. 8A) . In vitro data and a pharmacoscintigraphic study involving 6 patients have shown that whilst the enteric film coating safeguarded against drug release in the stomach, the HMPC coating ensured a reproducible lag phase once neutral pH values were encountered, providing drug release in the colon (Fig. 8B and C).The main limitation of time-dependent colonic delivery strategies is their reliance on predictable GI transit. As mentioned previously, GI transit is highly variable between and within individuals making accurate prediction very difficult, especially in disease states. This also applies to reliance on pH gradients as triggers to begin lag phases; where such gradients do not exist, there is more unpredictability.
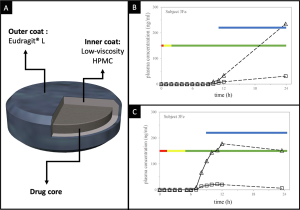
(A) Graphical illustration showing a 5-ASA dual time-dependent coating system. Plasma concentrations of (h) 5-ASA and (D) N-acetyl5-ASA following administration of the dual time-dependent coating system to a patient in the (B) fasted and (C) fed state. Red, yellow and green bars represent gastric, small intestinal and colonic residence respectively; the blue bar represents disintegration. Partially reprinted with permission from . (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
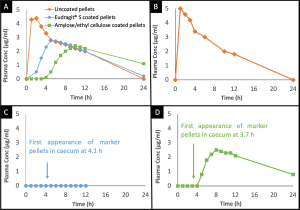
(A) Mean plasma theophylline levels after administration of uncoated, Eudragit_ S coated pellets and amylose/EC coated pellets to eight healthy male subjects. B, C and D show examples of plasma concentration time profiles observed in single subjects: (B) uncoated pellets; (C) Eudragit_ S coated pellets and; (D) amylose/EC coated pellets.
3-pH- and time-dependent combinations
The Multi Matrix System_ (or MMX_) is a system that combines a pH trigger mechanism along with a time-controlled release strategy . The drug, i.e. mesalazine [Lialda_ (Mezavant_ XL)] or budesonide [Cortiment_ (UCERIS_)], is incorporated in small lipophilic matrices which are embedded within a larger hydrophilic matrix. This ‘‘dual” matrix system is surrounded by an enteric film coating (e.g. based on Eudragit_ L and S). In theory, upon dissolution of the enteric coating at neutral pH, the ‘‘dual matrix” is expected to slow down drug release. Interestingly, although the release of 5-ASA was shown to be sustained for more than 8 h (upon the onset of initial drug release) and followed zero- order kinetics in phosphate buffer, the release patterns were substantially different in Krebs bicarbonate buffer]. In fact, 5-ASA release in bicarbonate buffer was more instantaneous and much more similar to that of ‘‘conventional” pH-sensitive colon targeting systems (e.g., Asacol_ and Ostasa_). Also in human subjects the MMX_ technology exhibited similar 5-ASA pharmacokinetics as a ‘‘pH-dependent, delayed-release mesalazine” . A similar example is represented by the Budenofalk_ product which is composed of granules with a dual coat; the inner layer is a mixture of Eudragit_ RS, Eudragit_ L100 and Eudragit_ S and the outer coat is comprised of Eudragit_ RL Another example is the Ethyl Cellulose Matrix (or ECXTM) which has been used within the multi-particulate dosage product EntocortTM EC. The system is designed to deliver the corticosteroid budesonide to the ileo-colonic region to treat CD and UC. Here, the system consists of a hard gelatine capsule containing 3 mg of the drug in pellet form. The pellets have an inert saccharose core coated with an inner layer of insoluble EC and an outer layer of Eudragit_ L100-55 [76]. Human pharmacoscintigraphic studies reported that a significantly greater fraction of budesonide was delivered to the ileo-colonic region, compared with the standard capsules . In addition, phase III clinical trials showed that clinical remission was significantly higher for patients taking EntocortTM EC compared with the placebo group . Recently, Eudratec_ COL was developed. This system is composed of an outer layer of Eudragit_ FS 30D coating and an inner layer of Eudragit_ RL or Eudragit_ RS. The latter two substances contain quaternary ammonium groups with chloride counter ions, enabling them to behave as diffusion-controlled membranes In vivo evaluation of caffeine pellets coated with an inner layer of Eudragit_ RL/RS 30D and an outer layer of Eudragit_ FS 30D reported ileo-caecal delivery, with a prolonged serum caffeine profile, compared with a pH-only system.
4-Time- and microbiota-dependent combinations
Formulations harnessing time and enzyme sensitive colonic delivery formulations have only recently begun to be developed. One such example exploited the combination of high amylose starch and HPMC of different chain lengths . The HPMC starts to swell and degrade when in contact with GI fluid, generating the lag time to reach the colonic region. Once the capsule reaches the colon, enzymes produced by colonic bacteria digest the starch portion, leading to a fast and complete release of drug cargo. These capsules have been loaded with paracetamol, and in vitro methods demonstrate faster pulsatile drug release when the formulation is in the presence of faecal microbiota. This highlights how both elements of the release system play an instrumental role in drug delivery and the synergy that results from the combined approach.
5-pH- and microbiota-dependent combinations
An example of a sequential pH and bacteria-sensitive system is the multilayer structure CODESTM . The tablet core consists of lactulose surrounded by Eudragit_ E coated with Eudragit_ L. After ingestion, the outermost Eudragit_ L coating protects the tablet from the acidic gastric fluid, and subsequently dissolves upon entry to the small intestine. Once in the colon, the lactulose component of the tablet begins to be fermented by bacteria. SCFAs produced during fermentation induces the dissolution of the acid-soluble Eudragit_ E coating, exposing the tablet core and prompting drug
release . Whilst sequential triggering systems combine more than one mechanism, in reality, they do not actually circumnavigate the risk of drug release failure nor provide a significant improvement in the reliability of the formulations. This is because the outermost layer will still be the rate-controlling step and if the critical threshold needed to trigger its dissolution is not reached in the patient’s gut, the inner matrix may not be triggered and the system is likely to exhibit variability in its drug release pattern.




